In today’s article, we will look closer at the structure of the US Healthcare system and also learn more about clinical trials. We will learn what the main purpose of clinical trials is, and how and what the clinical trials are conducted.
Healthcare as industry
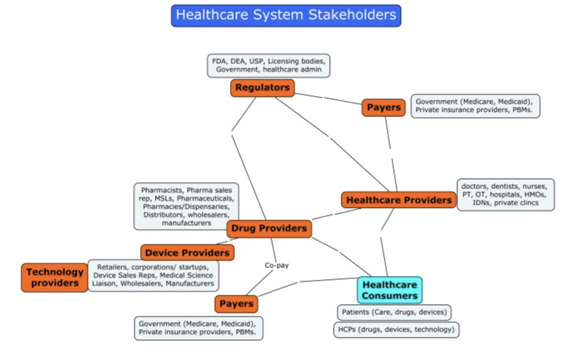
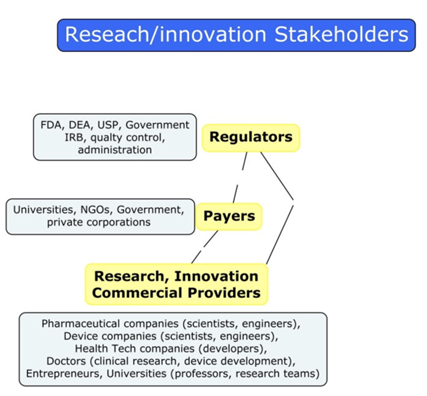
Healthcare as an industry has three main parties: Research and Innovation Providers, Payers, and Regulators. The major R&D providers are pharmaceutical companies, hardware and software HealthTech companies, clinics and hospitals, individual entrepreneurs and specialists, universities, and many others.
Federal agencies, quality control departments, university administrations, ethics review board
s, etc. can act as regulators.
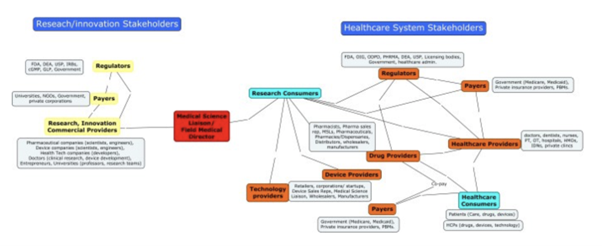
The Healthcare System players’ concept map
KOLS, MSLS, HCPS in Healthcare
KOLS
A KOL (key opinion leader) is usually an experienced physician trusted to give candid feedback to pharmaceutical suppliers.
They are often involved in the late stages of drug development as marketing consultants but they also contribute to the earlier phases.
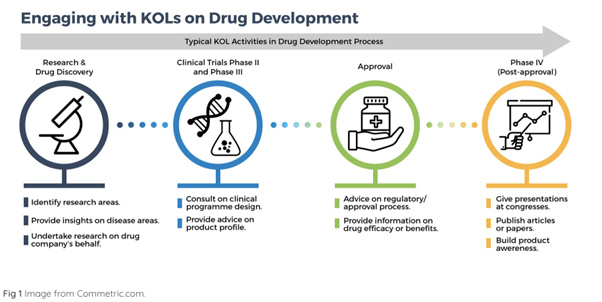
In the research and discovery phase KOLs:
- identify research areas, especially when there are sharp increases in hospital-acquired infections (HAIs);
- can offer insights on disease areas;
- research on behalf of drug companies.
In clinical trials KOLs:
- serve as a consultant on the program design;
- advise on the product profile.
In the approval phase KOLs:
- advise on the regulatory process;
- advise on the product profile.
In the post-approval and distribution phase KOLs:
- can build product awareness;
- publish articles or papers in medical resources;
- give presentations and promotions in congresses.
MSLS
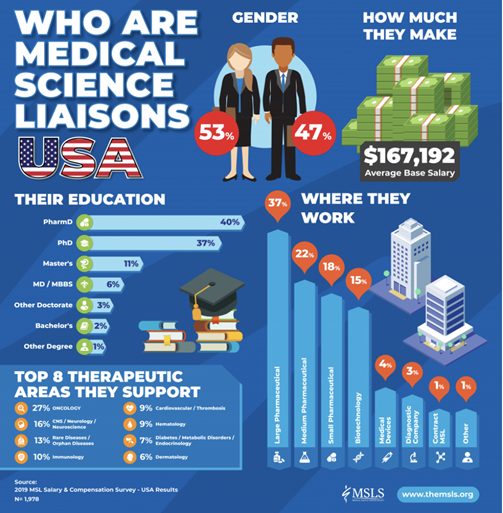
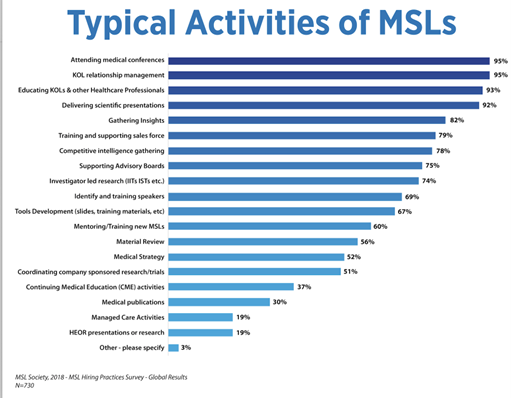
Medical Science Liaison (MSL) teams are made up of individuals with various scientific backgrounds. The MSL is an expert, who has experience in several therapeutic areas. In the US, the MSL can be f.e. a Pharmacist or a Medical doctor.
HCPS
Health care providers (HCP)/systems: Licensed practitioners (Doctors, dentists, nurses, physiotherapists, occupational therapists, etc.) all take direct care of their patients. They possess unique specialized expertise for which they are compensated appropriately.
HCPs offer their services in such environments as Private clinics, Hospitals, at the patient’s home, telehealth services, etc. Some of these practice groups may be linked together as a network because they have a single-payer; this is a Health Maintenance Organization. Patient costs are covered by the HMO (health insurance provider), often linked to the patients’ employers.
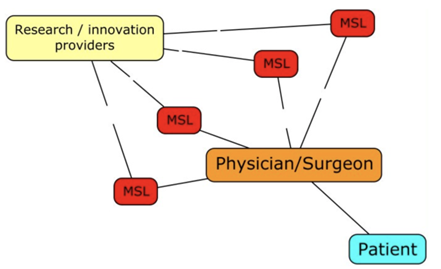
MSL-HCP INTERACTION (KOLS)
CLINICAL TRIALS
A clinical trial is a phase of research during which a new treatment is given to some participants to establish how safe and effective it is. Participants in clinical trials are divided into groups. One or more groups are control groups ― they are given no treatment, fake treatment known as a placebo, or regular treatment that patients with the studied condition usually receive.
Under double-blind conditions, neither the participants nor the clinical investigators know who is receiving the drug and who is receiving the placebo until the trial is complete.
PHASE 0
Usually, trials go straight to Phase I but sometimes a Phase 0 study will be run involving a small group of participants who are given a mini-dose of a drug.
The main aim of Phase 0 is to speed up the development of promising new drugs. Testing some drugs in very small doses in humans gives a clearer idea of drug-target interactions.
PHASE 1 or First in human (FIH)
This phase involves a small group (10-30 people) of healthy participants who receive payment.
A key principle for dose escalation in Phase 1 trials is maintaining rapid dose-escalation to avoid exposing too many patients to sub-therapeutic doses while preserving safety by limiting the frequency of toxic events.
PHASE 2 or Proof of concept (POC)
It involves a larger group of people compared to Phase 1 (50- to hundreds). At this phase, the new treatment is tested on people who have the condition that’s being targeted by the therapy. To ensure objectivity, the studies are randomized and double-blinded.
Phase 2 has certain metrics which are agreed upon with FDA (Food and Drug Administration) or EMA (European Medicine Agency) in advance.
This phase has primary and secondary endpoints. In case the treatment does not comply with the agreed metrics or proves toxic, the testing is interrupted. These studies may take up to two years.
PHASE 3
These trials are similar to Phase 2 trials but on a much larger scale (hundreds to thousands of participants).
Head-to-head trial – when the experimental drug is compared not only to the placebo but also to the regular treatment. The task is to prove that the experimental drug is the best.
Double-blinded – when neither doctors nor patients know which treatment is used. Secret tests with a very low fraud level.
Phase 3 studies collect large amounts of information about the treatment’s safety and effectiveness. Once these trials are completed, the treatment may be approved for general use.
This phase may take several years as well.
PHASE 4
This phase involves post-marketing trials. It uses feedback from medical practitioners to evaluate the treatment’s long-term safety. At this phase, researchers find out more about the side effects and safety of the treatment. It improves understanding of the long-term risks for patients and benefits of the treatment and shows how well the drug works when it is used more widely.
Summing Up
Healthcare as an industry is delivered through thousands of unique corporate entities, each of varying sizes, each involving interconnections. There are essential bidirectional relationships amongst various industry members. For Elinext, as for a HealthTech provider, it’s essential to understand these relationships deeply and the role of each party while developing a HealthTech product that is complied with all the standards and demands of the client and the end-user.